Products

ARMS Medical offers two innovative tissue products, DermaPure® and VNEW® using patented dCELL® Technology
dCELL® Technology removes more than 99% of DNA, leaving an intact extra cellular matrix.
What is dCELL® Technology?
Unlike alternative processes, dCELL® Technology results in greater than 99% DNA removal and produces decellularized biological tissue with virtually no structural disruption, thus providing an optimal replacement for the damaged extracellular matrix, allowing the patient’s living, native cells to populate and integrate ultimately achieving the goals of soft tissue healing and regeneration.
VNEW® represents the latest advancement from Dermapure, featuring pre-shaped decellularized dermal allografts tailored for anterior and posterior applications.
DermaPure Reimagined: Elevate Your Surgical Precision with VNEW® – The Pinnacle of Allograft Innovation.
VNEW® Pre-cut Decellularized Dermal Allografts For Pelvic Floor Surgery
Dermapure has taken a leap forward, now available in pre-cut shapes with the VNEW®. This is a next generation decellularized dermal allograft for pelvic floor surgery, processed with dCELL® Technology.
The VNEW® Pre-cut Decellularized Dermal allograft is the new evolution of VNEW®, and a non-oriented dual dermis allograft available in two precut shapes: anterior and posterior.
VNEW® is intended to be used for the repair, replacement, or reconstruction of damaged or inadequate integumental
tissue in the pelvic floor, including gender affirmation procedures.
This evolution to the original Dermapure also provides supplemental support to soft tissue (ligament) repairs in anterior, posterior, and apical prolapse procedures.

by

DermaPure® Decellularized Dermal Allograft
DermaPure® is a decellularized dermal tissue with virtually no structural disruption allowing tissue and cellular regeneration to occur. DermaPure® has been demonstrated to provide higher pro-angiogenic response during integration with reduced fibrosis.1,2
DermaPure® is available in three sizes:
3cm x 4cm, 4cm x 6cm, and 7cm x 10cm.
Reference:
Greaves NS, Iqbal SA, Morris J, et al. Acute cutaneous wounds treated with human decellularised dermis show enhanced angiogenesis during healing. PLOS ONE. 2015;10(1):e0113209. Greaves NS, Bayat A (2015). Skin Substitute-Assisted Repair Shows Reduced Dermal Fibrosis in Acute Human Wounds Validated Simultaneously by Histology and Optical Tomography. Wound Rep Reg (2015) 23 483–494

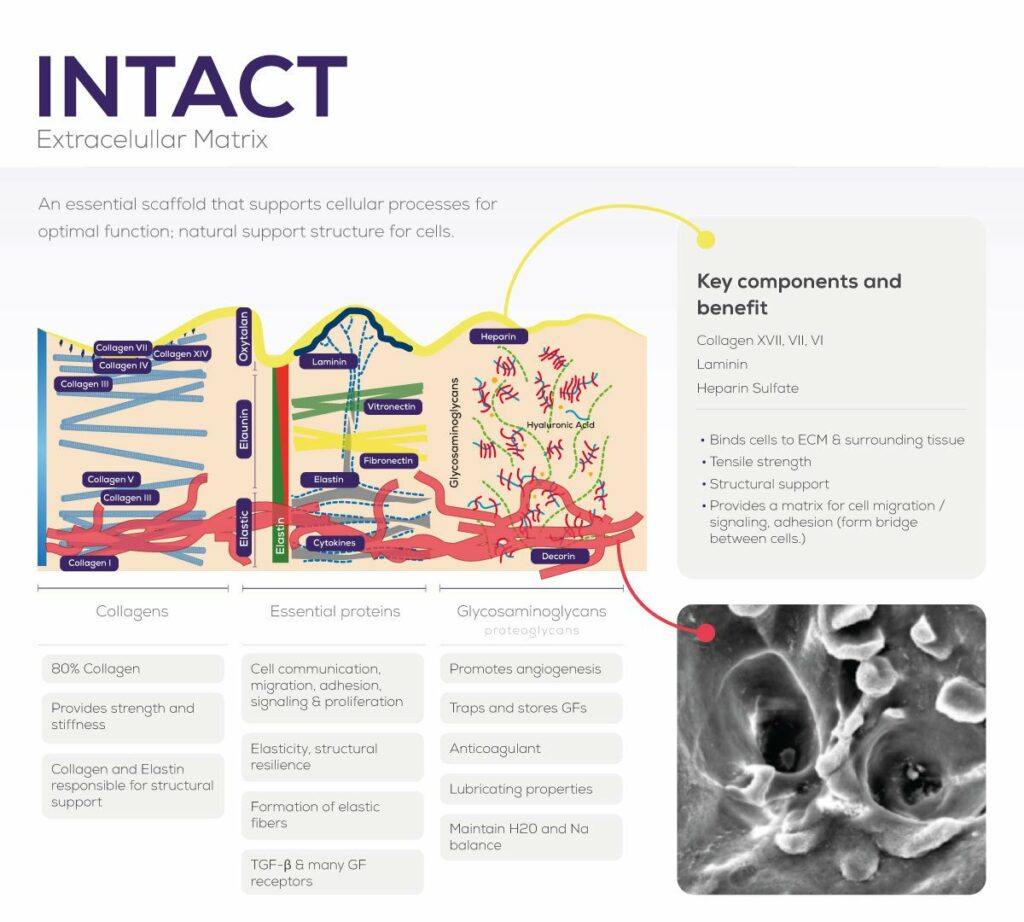
Tissue with dCELL® Technology is an intact extracellular matrix, with vascular-like channels necessary for revascularization.
Retains collagens, proteoglycans, glycosaminoglycans, as well as other extracellular matrix proteins.
Not all decellularization and tissue processing are the same…
Your patients are looking for new approaches to pelvic floor surgery that avoid the well-known complications of synthetic mesh, the scarring associated with native tissue repairs and the frequent post-surgery follow-up of issues resulting from both methods.


Among the human acellular dermis products being used in pelvic floor surgery, there are major differences in decellularization processes.
How are DermaPure® and VNEW® different from other allografts?
DermaPure® and VNEW® set itself apart from other tissue options and decellularization processes by using the unique and patented dCELL® Technology.
The result is lower immunogenicity because the decellularized donor tissue has nearly no structural disruption, with greater than 99% of the donor DNA removed.
How does DermaPure® perform?
- Facilitates the re-establishment of vascular channels, providing an access point to signal cellular activity enabling successful graft take in just seven days.(1)
- DermaPure® exerted the strongest pro-angiogenic influence, particularly within 3 - 4 weeks.(1)
- Treatment with DermaPure® resulted in reduced fibrosis compared to the control and xenograft.(2)
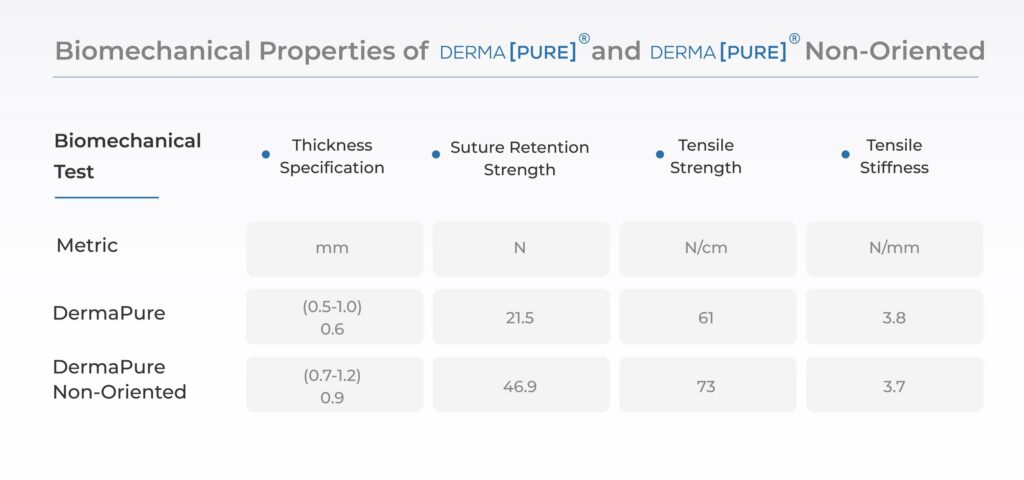
- Optimizes graft handling and suturability for surgeons retaining strong biomechanical properties in a thin profile decellularized dermal allograft.(3)
References: 1. Greaves NS, Iqbal SA, Morris J, et al. Acute cutaneous wounds treated with human decellularised dermis show enhanced angiogenesis during healing. PLoS ONE. 2015;10(1):e0113209. 2. Greaves NS, Bayat A (2015). Skin Substitute-Assisted Repair Shows Reduced Dermal Fibrosis in Acute Human Wounds Validated Simultaneously by Histology and Optical Tomography. Wound Rep Reg (2015) 23 483–494 3. Data on file at Tissue Regenix Limited.

DermaPure® has been used in over 3,000 pelvic floor procedures with no reported adverse events.*
Surgeon Advantages:
Flexibility: Suitable for abdominal, trans-vaginal and robotic surgery.
Enhanced angiogenesis and integration: DermaPure® exerted a higher quantity and quality of revascularization within 3 – 4 weeks and integrates with and closely approximates the structure and function of native tissue.1,2
Reduced fibrosis: Progressive colonization of DermaPure® by native cells results in expression of key genes that are associated with reduced inflammation.2
Reduces OR Time: Available in multiple sizes and precut shapes for posterior and anterior repair.
Safety: Provides a decellularized human tissue option, with an intact ECM, that is > 99% DNA free.
Ease of use: Optimizes graft handling and high suture pullout strength.
Video: Novel surgical approach incorporating DermaPure® with
the SSL and USL to address apical prolapse.
dCELL® Technology removes more than 99% of DNA, leaving an intact extra cellular matrix.
Vaginal Retractor System™
Vaginal Retractor System™ provides a safe and stable retraction in the narrow vaginal operative field for optimal visibility.
“Working closely with ARMS Medical as our provider of specialty pelvic floor surgical solutions, my team had the opportunity to use DermaPure allograft, along with the Veronikis Vaginal Retraction System. This solution allowed a minimally invasive vaginal approach for a positive surgical and patient outcome.”
— R. Keith Huffaker, MD, MBA, FACOG, FPMRS
Unlike alternative processes, dCELL® Technology results in greater than 99% DNA removal and produces decellularized biological tissue with virtually no structural disruption, thus providing an optimal replacement for the damaged extracellular matrix, allowing the patient’s living, native cells to populate and integrate ultimately achieving the goals of soft tissue healing and regeneration.
Vaginal Dilator Set
Vaginal Dilator Set with graduated couplers enabling patient to self-adjust to maintain their vaginal length.
Unique vaginal dilator with graduated couplers enabling patient to self-adjust to maintain their vaginal length
A versatile and adjustable dilator based upon patient’s needs
Provides two different length bases for seated and nighttime dilation
Graduated couplers allowing conversion to different sizes
Variable dilator lengths available from 1.5 cm to 13 cm
Vaginal dilators are designed to assist women in the treatment of the following symptoms:
Vaginal tightness
Painful intercourse due to pelvic floor dysfunction caused by (but not limited to) vaginal atrophy, vaginismus, vulvodinia, vulvar vestibulitis, interstitial cystitis, dyspareunia, vaginal narrowing and similar gynecological conditions
Vaginal discomfort following childbirth, post-surgical recovery or post radiation adhesions
Smoke Evacuation Pencil (SEP)
Smoke Evacuation Pencil (SEP) helps to reduce surgical smoke using superior design technology. All tips work with our ARMS Medical hand-piece so you can have the exact tool and precision you need.
Protecting patients and surgeons:
The SEP for removal of smoke and liquid during electrocauterization captures up to 150 different chemicals during the procedure.
VNEW Dermapure 2.0 FAQs
VNEW is an evolution of Dermapure, specifically designed as a pre-cut decellularized dermal allograft for pelvic floor repairs. While Dermapure offers a groundbreaking approach to tissue regeneration, VNEW enhances this with pre-cut shapes for anterior and posterior applications, leveraging advanced dCELL® Technology for even more precise and efficient surgical outcomes.
VNEW is intended for a broad range of pelvic floor repairs, including but not limited to the repair, replacement, or reconstruction of damaged or inadequate integumental tissue. This includes surgeries for anterior, posterior, and apical prolapse repairs, making it a versatile choice for addressing various pelvic floor conditions.
Yes, VNEW can be suitable for individuals who have undergone gender affirmation surgery and require pelvic floor repair or reconstruction. Its adaptability and the use of decellularized dermal allografts make it a potentially beneficial option for supporting soft tissue repairs in a wide array of surgical applications, including gender affirmation procedures.
The long-term benefits of VNEW include enhanced tissue integration, reduced risk of immune rejection, and improved surgical outcomes. Its advanced dCELL® Technology promotes natural tissue regeneration and healing, offering stronger, more flexible repairs that closely mimic natural human tissue behavior. This can lead to better durability, function, and overall quality of life post-surgery compared to traditional methods.
The recommended rest and recovery time after surgery using VNEW can vary depending on the individual’s overall health, the complexity of the surgery, and specific procedural details. Generally, VNEW is designed to enhance healing and potentially shorten recovery times thanks to its efficient integration with the patient’s own tissues. However, it’s crucial to follow personalized post-operative care instructions provided by your healthcare provider for the best outcomes.