The Gold Standard in Mesh-Free Repair
We’re here to help

Patient Resources
Suffering from pelvic organ prolapse or stress urinary incontinence? We’re here to connect you with information and treatment options.

Physician Resources
Medical professionals may request additional information, product specification sheets or connect with a VNEW® product specialist.

Products
Custom GYN instruments, pessaries, dilators and more.

- Common Conditions Treated Using VNEW®
- Cystocele
- Rectocele
- Synthetic Mesh Removal
- Enterocele
- Apical Prolapse
- Fistula
- Rectal Prolapse
- Stress Urinary Incontinence
- Complex Gender Procedures
- Cervical Cancer
Pioneering Mesh-Free Solutions
for Women’s Pelvic Health

- Common Conditions Treated Using VNEW®
- Cystocele
- Rectocele
- Synthetic Mesh Removal
- Enterocele
- Apical Prolapse
- Fistula
- Rectal Prolapse
- Stress Urinary Incontinence
- Complex Gender Procedures
- Cervical Cancer
Better tissue, better option.
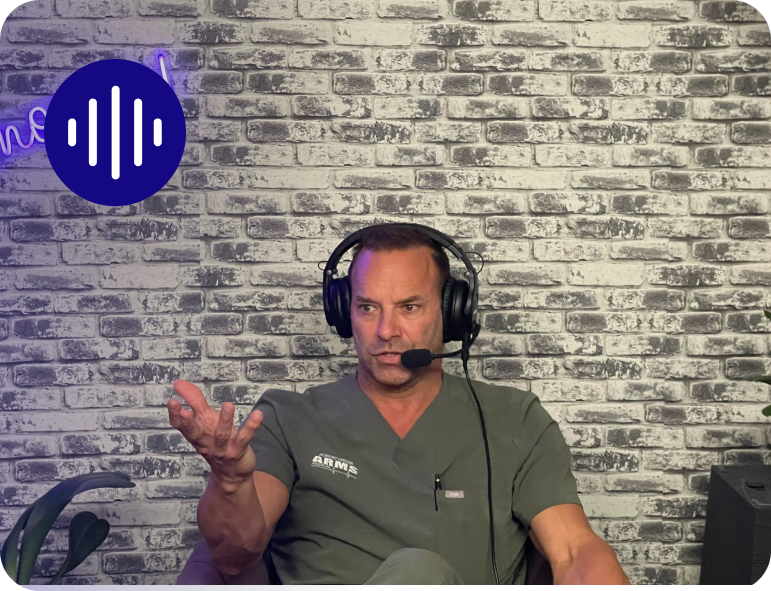
A Podcast for Pelvic Health Patients, Physicians & Hospitals
Explore innovative approaches to pelvic organ prolapse surgery. Discover the latest surgical techniques used by leading urogynecologists.
The lifestyle you want, the science you deserve
Approximately 300,000 women in the U.S. undergo surgical procedures for pelvic organ prolapse (POP) every year.
95% of women who had POP surgery utilizing DermaPure® or VNEW® recommend the surgery.

Common Symptoms Experienced by Women With
Dropped or Leaky Bladder

Lower Back Pain

Recurring UTI

Pelvic Pressure

Painful Intercourse

Vaginal Pressure

Chronic Urge to Urinate
Suffering from symptoms
You are not alone. Find a treatment plan that works best for you.
You are not alone
Find a treatment plan that worksbest for you
Testimonials
“I wish for more women to be able to experience the amazing recovery that I’ve enjoyed and I am grateful there are clinicians out there like ARMS and products like DermaPure® so women like me can participate in our lives uninhibited and unencumbered by a medical condition. It’s truly been a miracle.”
- Christine V., Patient and Registered Nurse
“When they removed the mesh, the DermaPure® was deployed to completely re-construct my urethra. It’s as if this product also fixed not just the damage mesh had created in my body but also retro repaired with childbirth had done to my urethra and my bladder. It’s truly been a miracle.”
- Christine V., Patient and Registered Nurse
“DermaPure’s® lack of DNA and the presence of decellularized vascular channels for tissue perfusion reduces rejection with decreased inflammation, and the preserved channels facilitate tissue incorporation and healing.”
- Dionysios Veronikis, MD, FACOG, FACS
“The use of decellularized allograft allows for a more thorough repair of a pelvic floor hernia without tension and without artificial materials. In principle, this should decrease the overall recurrence rate of the prolapse and improve the symptoms of perineal laxity without the risk of foreign body erosion.”
- Ana Toker, MD FACS, FACSRS
“In instances such as Stage III prolapse, a biologic implant like DermaPure® allograft is needed to improve the quality of the patient’s native tissue. In my experience, women presenting with Stage III prolapse have attenuated tissue. If they only receive a native tissue repair, they typically will develop a reoccurrence.”
- Manish Patel, M.D, RPh, FACS, FPMRS
“Since learning about DermaPure® from ARMS Medical, I have been very impressed by its advantages over synthetic mesh and competitive biologics. It offers good handling characteristics, is easy to place, incorporates well into surrounding tissue and has a negligible exposure rate. The proprietary dCELL® technology promotes tissue growth and healing more effectively than cross-linked products. I believe in this unique biologic platform and its usefulness in pelvic organ prolapse and mesh removal surgery.”
- James Chivian Lukban, DO, FACOG, FACS, FFPMRS
“DermaPure® was chosen to repair the tissue defect as it provides a firm and elastic tissue support that stimulates the natural healing process and serves as a scaffold upon which host cells can regenerate and differentiate.”
- Ervin Kocjancic MD
“Working closely with ARMS Medical as our provider of specialty pelvic floor surgical solutions, my team had the opportunity to use DermaPure® allograft, along with the Vaginal Retraction System. This solution allowed a minimally invasive vaginal approach for a positive surgical and patient outcome.”
- R. Keith Huffaker, MD, MBA, FACOG, FPMRS
“I have implanted DermaPure® allograft in more than 350 pelvic floor procedures and have had zero complications. In comparison, it’s not unusual for other biologics to cause an initial immune reaction or demonstrate poor healing.”
- Nathan L. Guerette, M.D., FACOG, FAAFP, FPMRS
“I have been looking for a new biologic to replace synthetic mesh in pelvic floor surgery. I learned about DermaPure® human allograft from ARMS Medical and participated in the excellent training they provided. This training included observing anterior and posterior surgical procedures performed by Nathan Guerette, a highly respected urogynecologist. I also learned how the DermaPure® tissue is processed with dCELL® technology.”
- Kenneth Baker, MD
“In our healthcare delivery landscape there’s constant pressure for economic efficiencies. As a surgeon, time is my most precious commodity. Having the VNEW® graft precut into anatomical shapes, and packaged to optimize ease to prepare for suture stabilization saves me significant OR time.”
- Vincent R. Lucente, MD, MBA, FACOG, FPMRS
By submitting this form, you are consenting to receive marketing emails from: ARMS Medical, 4970 SW 52nd Street #324 Fort Lauderdale, FL 33324 . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact.
Stay in touch for the latest news from ARMS Medical
Reasons to contact us:
- Info on registering your implant
- Questions about product (patient or hospital)
- Learn who your rep is
Stay in touch for the latest news from ARMS Medical
By submitting this form, you are consenting to receive marketing emails from: ARMS Medical, 4970 SW 52nd Street #324, Fort Lauderdale, FL 33324 . You can revoke your consent to receive emails at any time by using the SafeUnsubscribe® link, found at the bottom of every email. Emails are serviced by Constant Contact.
Reasons to contact us:
- Info on registering your implant
- Questions about product (patient or hospital)
- Learn who your rep is
*Reference: NL Guerette, Connelly JR, C Jay, and JC Lukban. Safety and Anatomic Efficacy of Transvaginal Prolapse Repair Augmented With a Novel Decellularized Human Dermal Allograft. AUG 2019. dCELL® technology is a registered trademark of Tissue Regenix Limited.





